And Then There Were Three…: Extreme Regeneration Ability of the Solitary Chordate Polycarpa mytiligera. Tal Gordon et al. Front. Cell Dev. Biol., April 15 2021. https://doi.org/10.3389/fcell.2021.652466
Abstract: Extensive regenerative ability is a common trait of animals capable of asexual development. The current study reveals the extraordinary regeneration abilities of the solitary ascidian Polycarpa mytiligera. Dissection of a single individual into separate fragments along two body axes resulted in the complete regeneration of each fragment into an independent, functional individual. The ability of a solitary ascidian, incapable of asexual development, to achieve bidirectional regeneration and fully regenerate all body structures and organs is described here for the first time. Amputation initiated cell proliferation in proximity to the amputation line. Phylogenetic analysis demonstrated the close affinity of P. mytiligera to colonial species. This evolutionary proximity suggests the ability for regeneration as an exaptation feature for colonial lifestyle. P. mytiligera’s exceptional regenerative abilities and phylogenetic position highlight its potential to serve as a new comparative system for studies seeking to uncover the evolution of regeneration and coloniality among the chordates.
Discussion
Ascidians are unique among the chordates as they present robust regeneration abilities, including whole body regeneration, following an injury (Voskoboynik et al., 2007; Blanchoud et al., 2018; Kassmer et al., 2020). The current study has uncovered exceptional regeneration abilities in a solitary ascidian, a group of animals considered to be regeneratively deficient compared to their colonial relatives (Kassmer et al., 2019). P. mytiligera’s provides a valuable opportunity to elucidate the evolution of coloniality and reveal conserved pathways that regulate chordate regeneration.
P. mytiligera Regeneration Involves Extensive Cell Proliferation and Reorganization Processes
P. mytiligera presented an extraordinary regeneration plasticity, being able to reconstruct an entire animal from each small body fragment. Histological sections confirmed that despite no residue of essential organs, such as the heart and neural complex, remaining in the body fragment, the animal was nonetheless able to regenerate and regain its complete morphology and functionality.
P. mytiligera’s regeneration process can be divided into three phases: (1) wound-healing; (2) increased cell proliferation in the injured area; and (3) morphogenesis and formation of tissues and organs. These basic stages, underlying tissue regeneration, are highly conserved, being found in a wide variety of animals capable of regeneration (Alvarado and Tsonis, 2006; Ricci and Srivastava, 2018). In planarians, regeneration involves the assembly of a blastema composed of pluripotent somatic stem cells. Limb regeneration in salamanders and fin regeneration in zebrafish also require the formation of a blastema; however, there the blastema is composed of a mixture of cells with different and restricted potentials (Alvarado and Tsonis, 2006; Sánchez Alvarado, 2006; Tanaka and Reddien, 2011; Tanaka, 2016; Marques et al., 2019). In P. mytiligera, amputation initiated extensive cell division in proximity to the amputation line, resembling a blastema formation. Proliferating cell were also found in regenerating structures at later stages of regeneration, suggesting their direct involvement in the formation of the new tissue (see Figure 7 for summary). Undifferentiated circulatory cells were shown to be involved in tissue regeneration in solitary and colonial ascidians (Rinkevich et al., 2006; Auger et al., 2010; Jeffery, 2014, 2015, 2019). In colonial Styelidae species, a population of pluripotent or multipotent undifferentiated circulatory cells contributes to the formation of somatic tissues during budding and whole body regeneration (Laird et al., 2005; Voskoboynik et al., 2007; Brown and Swalla, 2012; Kassmer et al., 2020). In the solitary ascidian C. intestinalis, injury-induced regeneration resulted in the proliferation of circulatory cells located in the branchial basket. These cells were also labeled with alkaline phosphatase and anti-piwi antibody, indicating their undifferentiated state (Auger et al., 2010; Jeffery, 2014). In P. mytiligera, the branchial basket showed high level of EdU+ cells along the regeneration process. In addition, all dissected body fragments contained part of the branchial basket and the circulatory cells enclosed within it. While it is remained to be determined if these cells originated in the branchial basket or merely use it as a means of transport to the regenerating area, the high numbers of proliferating cells found in the branchial basket following amputation indicate a possible role in anterior regeneration.
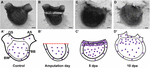
Figure 7. Summary of proliferating cell dynamic in P. mytiligera inferred from EdU experiments. (A,A′) Control. (A) In vivo image, (A′) illustration. During homeostasis, proliferating cells (purple spheres) are evenly dispersal along the branchial basket (BB) and body wall (BW) [Oral siphon (OS), atrial siphon (AS)]. (B,B′) Amputation day. (B) In vivo image, (B′) illustration. The level of proliferating cells remains similar to that of the control. (C,C′) 5 days following anterior amputation. (C) In vivo image, (C′) illustration. Proliferating cells specifically accumulate at the amputation site in the branchial basket and body wall in proximity to the regenerating area. (D,D′) 10 days following amputation. (D) In vivo image, (D′) illustration. As the amputated structures regenerated, the level of proliferating cell decreased in relation to 5 dpa, although remaining higher than those of the control. Scale bar in (A,B): 1 mm, and in: (C,D): 500 μm.
While we focused on the cell division in the body wall and branchial basket in the current study, proliferating cells were also found in other structures as the digestive system and endostyle. The digestive system of the control animals as well as in animals in different stages of regeneration showed EdU positive cells. These cells, however, are probably unrelated to the regeneration signals, as intestinal and stomach tissues are continuously being replaced as part of their normal homeostasis (Ermak, 1981; Jeffery, 2014). The endostyle, which serves as a stem-cell niche in colonial species (Voskoboynik et al., 2008), showed EdU labeling at the early stages of regeneration, implying a local cell proliferation and its possible involvement in regeneration. However, it is important to note that the results of the DV axis amputation experiment indicate that the endostyle is not essential for regeneration to occur, as the dorsal body part was still able to complete the regeneration process following endostyle removal.
Among ascidians, the ability to regenerate amputated body fragments into separate individuals is strongly associated with coloniality and asexual development, as no solitary species has to date been observed to possess such robust abilities (Jeffery, 2015; Kassmer et al., 2019). The model system, C. intestinalis, is capable of regenerating its anterior structures, such as the siphons and neural complex from the posterior parts; whereas the anterior parts failed to regenerate posterior structures such as the heart and digestive system, and eventually decomposed (Jeffery, 2014). P. mytiligera’s bidirectional regeneration and unusual ability to regenerate all tissue types and organ systems distinguishes it from other solitary ascidian species studied so far (see Supplementary Table 4 for summary) and suggests the activation of regeneration programs that might be compromised or inhibited in other solitary species (Liu et al., 2013; Sikes and Newmark, 2013).
P. mytiligera’s High Regeneration Abilities Might Constitute a Pre-adaptation (Exaptation) Trait for a Colonial Life-Style
P. mytiligera’s reproduction and developmental processes resemble those of most solitary ascidians and no indication of asexual development has been found for this species (Gordon et al., 2020). However, its ability to create “clones″ following dissection led us to further question its phylogenetic position.
The Stolidobranchia common ancestor is believed to have been solitary, and coloniality is assumed to be a derived life-style (Mukai et al., 1978; Zeng et al., 2006). The Styelidae is the only stolidobranch family composed of colonial and solitary species, with both presenting a wide range of developmental and regeneration processes (Alié et al., 2020). Phylogenetic analyses have indicated several independent acquisitions of coloniality in this group (Kott, 1985, 2005; Pérez-Portela et al., 2009; Alié et al., 2018, 2020). Further support for multiple transition events from a solitary to a colonial life-style comes from species that have diverged from the classical solitary or colonial characteristics, and which present intermediate morphological and developmental features. For example, solitary species of Polycarpa and Dendrodoa genera present colonial characteristics such as viviparity, a typical colonial feature (Millar, 1954, 1962; Svane and Young, 1989; Pérez-Portela et al., 2009).
Our phylogenetic results agree with Alié et al. (2018) and separate the Polycarpa genus from the exclusively solitary clade, placing it in a single mixed clade composed of colonial and solitary species. This topology suggests that the last common ancestor of the mixed clade was a solitary animal from which coloniality evolved at a later stage (Alié et al., 2018). According to this scenario, high regeneration abilities, as presented by P. mytiligera, might be a pre-adaptation (exaptation) trait for colonial life-style. The position of the colonial Polyandrocarpa zorritensis among members of the genus Polycarpa is especially intriguing, as it further supports the genetic similarity of Polycarpa to highly regenerative colonial species, as well as indicating a recent transition event from solitary to colonial form in this family (Alié et al., 2018; Scelzo et al., 2019).
To date, ascidian whole-body regeneration has been considered a colonial feature, as no solitary species had been shown before to possess such robust abilities. P. mytiligera’s ability to regenerate each individual body fragment into a whole animal has the potential to separate regeneration specific pathways from asexual development programs.
Our present findings present a valuable new model system for comparative developmental studies seeking to elucidate the evolution of regeneration and coloniality among the chordates.
Data Availability Statement
The datasets generated for this study can be found in the online repositories. The names of the repository/repositories and accession number(s) can be found below: Sequence data have been deposited under: https://www.ncbi.nlm.nih.gov/bioproject/660913. The phylogenetic datasets are available at: https://github.com/dorohuchon/Polycarpa_mytiligera_transcriptome. The maximum likelihood trees, the alignments, and the transcriptome assembly have been deposited at https://github.com/dorohuchon/Polycarpa_mytiligera_transcriptome.



No comments:
Post a Comment